Volatile Production and Ground Beef and Reaction

Volatile Profile of Dry and Wet Anile Beefiness Loin and Its Relationship with Consumer Flavor Liking
one
Faculty of Veterinarian and Agronomical Sciences, School of Agriculture and Nutrient, The University of Melbourne, Parkville, VIC 3010, Australia
2
Commonwealth Scientific and Industry Enquiry Organization, 11 Julius Ave, North Ryde, NSW 2113, Australia
3
School of Environmental and Rural Scientific discipline, Academy of New England, Armidale, NSW 2351, Australia
*
Author to whom correspondence should exist addressed.
†
Present Address: Center for Advanced Nutrient Engineering, The Academy of Sydney, Sydney, NSW 2006, Australia.
Academic Editor: Baohua Kong
Received: 9 Nov 2021 / Revised: 9 Dec 2021 / Accepted: x Dec 2021 / Published: xv December 2021
Abstract
This written report investigated the effect of ageing method and ageing time on the volatile profiles of grilled beef striploins (Longissimus thoracis et lumborum) and their relationship with consumer flavour liking. Volatiles were measured in grilled steaks subjected to 35 days of dry ageing, 35 days of wet ageing, 56 days of dry ageing or 56 days of wet ageing, using headspace-solid-stage microextraction followed by gas chromatography-mass spectrometry. Gas chromatography-olfactometry-mass spectrometry was besides conducted on 35-twenty-four hour period wet and dry aged samples to identify volatiles with loftier odour touch on. The concentration of many odour touch volatiles, e.m., 3-hydroxy-two-butanone, two-acetyl-2-thiazoline, and various alkyl-pyrazines, was significantly college in dry aged beefiness compared to wet aged beefiness (p < 0.05). Several odour bear upon volatiles, e.g., 2-acetyl-one-pyrroline, and alkyl-pyrazines, decreased significantly with ageing time (p < 0.05), while volatile products of lipid oxidation and microbial metabolism increased with ageing time. Partial to the lowest degree-squares regression analysis showed that the college consumer flavour liking for 35-day dry out aged beef was associated with higher concentrations of desirable scent-active volatiles.
1. Introduction
Ageing in vacuum packs is widely used in the beef industry to preserve and to improve the eating quality of meat [ane]. Ageing of beef is most commonly achieved by the storage of primals or steaks in vacuum packaging under controlled depression temperature to improve the tenderness and extend the shelf life [2]. This process is usually termed 'wet' ageing. In contrast, 'dry' ageing of beefiness refers to the ageing of unpackaged primals in air nether strictly controlled weather including temperature, moisture, and air velocity [iii]. Dry out aged beef is often marketed as a premium product with improved flavour by loftier-end butchers and restaurants, although it remains controversial whether the sensory quality of dry aged beef is college than that of wet aged beef [four]. A more recent written report showed that dry out anile beefiness received significantly college overall and flavour liking scores compared to wet aged counterparts using Meat Standard Australia (MSA) consumer panels with 1440 consumers [3]. Although both ageing methods improve most palatability attributes of beefiness, moisture aged meat was reported to be associated with negative flavours including sour, 'serumy', and metallic, whereas the dry ageing is known to enhance the positive flavours, such every bit nutty, roasted, and buttery, in beef [5].
Due to the consensus amid consumers and retailers that dry ageing significantly improves the season of beef compared to that of wet ageing, the season chemistry of dry anile beefiness has been a subject of investigation in several studies [4,vi]. Iida, et al. [half-dozen] showed that the deviation in flavour between dry and wet aged beef could be partially attributed to the increase in umami taste of dry aged beef. While nearly studies of dry/wet ageing of beef were focused on the sensory cess and the taste-active compounds, the role of season-active volatiles in the flavour of aged beef is not well understood. Volatiles formed in cooked beef have been proven to play an essential office in the perception of flavour [vii,8]. The characteristic aroma of cooked beefiness is largely due to volatile substances formed during cooking [9]. King, et al. [x] and Utama, et al. [11] reported that the volatile profile of dry anile beef is unlike from that of wet aged beefiness. However, the human relationship betwixt sensory flavour liking, and volatiles was not explored in their research. Bated from the ageing method (dry or wet), the period of ageing is another determinant of the palatability of aged beef. Dry ageing of beef for more than 40 days is reported to negatively impact on eating quality due to increased lipid oxidation and microbial spoilage [3,6]. The influence of ageing time on volatiles in cooked, moisture aged beef was shown in the report of Frank, et al. [12] and Watanabe, et al. [13]. While the alter of volatiles in dry ageing of beef and its comparing with moisture ageing are rarely reported.
The main goal of this study was to analyse the effects of dry and wet ageing, and ageing time, on volatile profiles of beef. The relationship between volatiles and flavour liking of aged beef was too investigated to explain the departure in season liking of beef anile with different ageing methods.
2. Materials and Methods
ii.1. Animal and Carcass Collection
The sample drove process for this project was described fully in the study of Ha, et al. [3]. The cattle were mixed breeds of predominantly Angus, Hereford and Murray Gray, <24 months one-time and were hormone- and antibody-free. Briefly, carcasses (due north = 24) from 16 steers and eight heifers with normal pH (<5.seven) were selected at 24 h post mortem from a commercial beefiness processing plant in Tasmania, Australia. Full length bone-in longissimus thoracis et lumborum was excised from both sides of 24 carcasses. All primals were vacuum packed and transported in a refrigerated lorry to Top Cut Foods (Gold Declension, QLD) for further processing and ageing.
2.two. Ageing Specification
The wet and dry ageing conditions were described in the study of Ha, et al. [iii]. Upon arriving at Summit Cut Foods, primals were boned into striploins (boneless) or OP (Oven Prepared) ribs (bone-in). The ageing method by ageing combinations were allocated within 2 longissimus thoracis et lumborum primals from ane carcass. There were seven combinations of treatments, and only the 35-day wet and dry aged, and the 56-day wet and dry aged samples from 12 randomly selected carcasses were used in the present study. Wet ageing was conducted with boneless primals in Cryovac® polyamide, polyethylene vacuum pack bags with an oxygen transmission rate of 20 cc/chiliad2/24 h at 23 °C without illumination. The primals were placed in a refrigerated room with temperature fluctuating betwixt 2–6 °C.
The dry ageing room was a multi-batch chiller with movable racks and two UV lights fitted to its ceiling. The meat samples were rotated daily to different positions in the chiller. The relative humidity (RH) in the bedchamber ranged from 53% to 100% with an boilerplate RH of 89.4% over the experimental period. The recorded temperature was 1.3 °C to iv.ii °C with an average temperature of 2.1 °C. The airspeed at the central position varied between 0.75 and 1.ii m/s.
After each ageing menstruation, steaks 2.five cm thick, and pocket-sized samples, were obtained from the primals and frozen at −20 °C for 3 months until sensory analysis or at −eighty °C for four months until flavour chemistry assay. Consumer flavor liking was obtained using Meat Standards Australia (MSA) untrained consumer panels with 900 participants equally described by Ha, et al. [3].
2.3. Intramuscular Fat Analysis
Intramuscular fat (Imf) was measured using nearly-infrared spectrometry as described by Perry, et al. [fourteen]. Frozen samples (approximately 100 one thousand) were freeze-stale and finely ground. The ground samples were analysed with a Technicon InfrAlyser 450 spectrometer (Bran and Luebbe, Sydney, Commonwealth of australia) and expressed as percentage of Imf in raw meat (w/w).
2.four. Headspace-Solid-Phase Microextraction Gas Chromatography-Mass Spectrometry (HS-SPME GC-MS)
Beef steaks were thawed overnight at 4 °C before testing and grilled for 3 min using a clamshell grill (Silex, Marrickville, Australia) set at 220 °C. The samples were then allowed to rest for another 3 min nether aluminium foil. The grilled beefiness steaks were then weighed, roughly cutting and Milli-Q h2o was added at a ratio of 1:ii (Milli-Q h2o:meat). Samples were macerated using a hand-held food processor and iv k of slurry was transferred to a headspace vial. The internal standard 4-methyl-1-pentanol was placed into a 200 µL insert. Duplicate samples were placed in the autosampler (AOC-5000, Shimadzu, Rydalmere, Australia). The samples were pre-incubated at 40 °C for 15 min and the headspace volatiles were extracted with divinylbenzene/carboxen/PDMS 23-gauge, 2 cm solid phase microextraction (SPME) fibres (Supelco, Sigma-Aldrich, Castle Hill, Australia) for 40 min at forty °C with agitation. The extracted volatiles were desorbed in splitless mode into a hot injector (250 °C) for 5 min and separated using gas chromatography–mass spectrometry (QP-2010-Plus GC-MS, Shimadzu, Rydalmere, Australia) on a Zebron- Wax cavalcade (Phenomenex, Lane Cove West, Commonwealth of australia, thirty m, 0.25 id, 0.25 μm film) with the following temperature programming; initial temperature of 35 °C was held for 5 min and then heated at 5 °C/min to 250 °C. The electron impact (EI) mass spectrometer was programmed to scan the mass range m/z 40–250. An aliphatic hydrocarbon mix (C8-C32, Sigma-Aldrich, Castle Colina, Australia) was used to determine linear memory indices. Compounds were identified by comparison their electron impact mass spectra with reference spectra in the National Institute of Standards and Engineering science mass spectral database (NIST 2002) and by linear memory indices matching those of published values (NIST Chemistry WebBook). Integrated area data were normalised to the internal standard and semi-quantitative data (µg/g) were estimated.
ii.5. Gas Chromatography-Olfactometry-Mass Spectrometry (GC-O-MS)
GC-O-MS analysis was conducted on a subset of 35-twenty-four hours dry out aged and moisture aged samples (n = 8 carcasses). A panel of trained GC-O assessors (n = 6) evaluated the column effluent of each matching pair (from the aforementioned carcass) of wet and dry out aged samples using a validated directly intensity technique. Assessors were trained according to previously reported protocols [xv]. Volatiles were trapped onto Tenax traps and desorbed using a brusque path thermal desorption unit (Scientific Services, Ringoes, NJ, U.s.a.) onto the injector port of the GC-O-MS and separated on a Zebron-Wax capillary column as described previously [8]. Briefly, assessors measured the smell intensity of the GC effluent using a figurer mouse and time intensity software SensoMaker [sixteen]. The scent intensity throughout the chromatographic run (approximately twenty min) was rated continuously using an unstructured 10 cm line scale on a calculator screen, where 0 represented the absence of any perceived odour, 2.5 was used to betoken an scent of mild intensity, 5.0 moderate intensity, 7.5 stiff and 10 very strong. Odour intensity data were continuously caused at a rate of 1 Hz. In case of odours persisting for several seconds, assessors were asked to continuously charge per unit the intensity until the aroma stimulus disappeared. Simultaneously, assessors were asked to verbally draw the olfactory property quality using a microphone. Assessor descriptions were digitally recorded (GoldWave Inc., St John's, NL, Canada). Time intensity data from each panellist were imported into Microsoft Excel and annotated with odour descriptors (when given) and matched to specific volatiles based on compounds identified eluting at the same time; for example, from the electron bear on mass spectral data in National Constitute of Standards and Technology library. For each distinct odour effect, the integrated area under the time curve (AUC) was calculated, for case intensity (1–10) × duration (seconds). Replicate AUC data were used for statistical analysis and the average AUC was used to construct aromagrams. Peaks detected by less than two assessors were considered dissonance and deleted from the aromagram. As in that location was no time delay between the GC-MS and the olfactory port effluent, odours and volatiles could be accurately matched. Retention indices were calculated for volatiles on the GC-O to enable cantankerous-referencing to the SPME data.
2.6. Statistical Assay
Volatile information were analysed using GenStat® (16th Edition, VSN International, Hemel Hempstead, Uk). The multivariate assay of variance (MANOVA) was performed on the volatile data using the following model; Response (volatile data) = ageing method (principal event) + ageing fourth dimension (main effect) + ageing method ∗ ageing time (interaction) + animal (block) + intramuscular fat content (covariate). The boilerplate standard error of difference (SED) were calculated for chief effects and interactions. Effects of treatments were considered significant if the deviation between treatments were greater than 2 × SED.
The consumer flavour liking scores reported in Ha, et al. [3] were matched with mean integrated volatile data from samples from the same carcass and handling group (due north =12 carcasses in each ageing method ∗ ageing time treatment group, in that location was one carcass with missing sensory data in 56-day dry ageing and one in 56-solar day moisture ageing groups respectively). Principal component assay (PCA) was performed on flavour liking information of samples and their respective semi-quantitative volatile data with confirmed odour impact in GC-O analysis or literatures using Matlab (R2019b, The MathWorks Inc., Natick, MA, United States). A measure of sample adequacy was also performed using the Kaiser–Meyer–Olkin (KMO) test to check the suitability of the selected variables for PCA. A fractional least-squares regression analysis was performed on the mean-integrated volatile and sensory data for each treatment grouping using the fractional least-squares (PLS) procedure in Genstat post-obit the method described by Frank, et al. [15]. All data were first standardised using the z-score function in Matlab. All volatiles results were used in the initial model, and the X-component loadings were used to select the almost influential volatiles for flavour liking of aged beef. A subset of volatiles (n = 22) known to be odour-active in the GC-O results or the literature were selected for the last optimised PLS model. One latent gene was used in modelling with cross-validation procedures (four groups, random seeds = 2). The Osten's F-test was performed on the predicted sum of square statistic to evaluate the significance of latent factor. The root hateful square fault of cantankerous validation was calculated from the predicted residual sum of foursquare statistic.
three. Results and Give-and-take
3.1. Grilled Beefiness Volatile Profiles
A total of 62 volatiles were identified in the grilled beef headspace extracts based on EI mass spectra and retention indices (Tabular array i). 2-Methylbutanal, ethanol, and 3-hydroxy-two-butanone were quantitatively the almost abundant volatiles. Hexanal, butyl formate, 3-methylbutanal, 2,half dozen-dimethylpyrazine, 2-pentanone, trimethylpyrazine, 2,5-dimethylbenzaldehyde, and 2-ethyl-3,v dimethylpyrazine were also measured at relatively loftier concentrations. Most of these volatiles accept been reported in beefiness odor extracts previously [8,17,xviii]. Among these compounds, 3-hydroxy-2-butanone was shown to exist associated with aged meat [18]. Pregnant differences were measured for several odour-active volatiles for the main effects of "ageing method" or "ageing fourth dimension" and their interaction (Table 1). Overall, the dry out aged and wet anile beef had similar intensity of total volatiles. A significantly college concentration of alcohols, especially ethanol, was measured in the moisture aged beef compared to dry anile counterparts, and the concentration of ethanol increased significantly over time in wet ageing. The increased ethanol in wet aged beef is likely to exist a upshot of lactic acid bacteria fermentation every bit reported previously in vacuum-packed beefiness [12,19,20]. Similarly, the concentration of acerb acid was significantly college in moisture aged beefiness. The finding is consequent with previous studies and the higher acetate could be explained by the fermentation of microflora [20]. It is worth noting that many other organic acids might also increase during vacuum storage, only they are not volatile and thus non measured hands by HS-SPME GC-MS. Esters are known to be formed in the esterification process in the cooking of beefiness or by microbial spoilage [21]. In the nowadays study, butyl formate was detected at a relatively loftier concentration in both dry aged and wet aged beef but decreased with the ageing time. Butyl formate is rarely reported in the headspace of grill beef, and a previous study indicates that it could be formed in an unusual microbial metabolism in the wet ageing of beefiness [22]. Ketones such as 3-hydroxy-2-butanone and 2-pentanone were significantly different between ageing methods. The concentration of 3-hydroxy-ii-butanone was significantly higher in dry aged beefiness than that in wet anile beefiness. The 3-hydroxy-ii-butanone is known as an oxidative product of saturated fat but has too been shown to be related to the Maillard reaction [23]. three-Hydroxy-2-butanone is considered an important correspondent to the buttery scent in beef [24]. The concentration of acetone increased significantly over fourth dimension regardless of the ageing method. The increase of acetone in the headspace of meat was also reported to exist associated with microbial metabolism during storage [25].
Aldehydes generally play important roles in the season of beef and quantitatively dominate many other odour-active volatiles in cooked beef [9]. In meat, aldehydes are mainly formed during lipid oxidation (due east.chiliad., hexanal, octanal, nonanal) and the Strecker degradation of amino acids, such as 2-methylbutanal, 3-methylbutanal and benzaldehyde [26]. In the present study, the concentration of two-methylbutanal and iii-methylbutanal did not differ with ageing method or ageing time, but the concentration of 2,5-dimethylbenzaldehyde decreased significantly with ageing time. The Strecker deposition of amino acids is primarily caused past its reaction with dicarbonyl compounds formed in the Maillard reaction [9]. In the ageing of beefiness, the concentration of free amino acids has been reported to increase continuously due to proteolysis [12,27]. Therefore, nosotros speculate that the lower extent of Strecker degradation of 56-twenty-four hours aged beef was probable due to lower availability of dicarbonyl compounds from the Maillard reaction [28,29]. This hypothesis is further supported past the lower extent of Maillard reaction in 56-day aged beefiness, every bit indicated past the lower concentrations of alkyl-pyrazines, compared to its 35-day aged counterpart measured in the nowadays report. Hexanal, heptanal, octanal, and nonanal, are unremarkably reported lipid-derived volatiles in cooked beef, and their odours are described as 'greenish', 'fatty', and 'sweetness' [30] and are usually used as the indicators of lipid oxidation in meat [31]. In our report, the concentrations of heptanal, octanal, and nonanal increased with the ageing time in dry ageing (p < 0.05 except for heptanal) but decreased with the ageing in moisture ageing (p < 0.05 for all). In the beef aged 56 days, the concentrations of these volatiles were college in the dry aged meat compared to the wet aged meat (p < 0.05). The increase of these volatiles in dry ageing agrees with the results of thiobarbituric acid reactive substances (TBARS) obtained in the same samples [3]. However, the TBARS in the wet anile beef increased with ageing fourth dimension in the study of Ha, et al. [iii], which is contradictory to the volatile results. Therefore, the relationship between volatile compounds and TBARS was not clear in the nowadays report, and a possible caption for this is the conversion of volatiles aldehydes into organic acids due to the microbial activities in the wet ageing [32]. Whereas the malondialdehyde measured by TBARS is unlikely to be affected by the microorganisms in meat during the moisture ageing.
Significant furnishings of ageing method and ageing time were measured for alkyl-pyrazine compounds in the present report (Table 1). Specifically, 2,v-dimethylpyrazine, 2,half dozen-dimethylpyrazine, 2-ethyl-v-methylpyrazine, two-ethyl-6-methylpyrazine, trimethyl pyrazine, 2-ethyl-iii,5-dimethylpyrazine, and 3-ethyl-2,five-dimethylpyrazine were detected at relatively high affluence. The concentrations of well-nigh of these compounds were significantly college in the dry aged beef compared to moisture anile samples (p < 0.05, except for two,five-dimethylpyrazine and two,half dozen-dimethylpyrazine), and the concentration tended to decrease with ageing fourth dimension in both ageing methods (p < 0.05, except for 2,half-dozen-dimethylpyrazine and trimethylpyrazine). This result indicates a higher rate of Maillard reaction in dry aged beefiness compared to wet aged. The difference in Maillard reaction could be attributed to the ultimate pH equally reported by Ha, et al. [3]. The dry aged beef had significantly higher pH compared to that of moisture anile beef subsequently ageing (p < 0.001), and the pH declined in both ageing methods with the ageing fourth dimension. The study of Madruga and Mottram [33] shows that the formation of alkyl-pyrazines during the cooking of meat is pH dependent, and a higher pH contributed to its generation. It was postulated in their studies that the unprotonated amino acids are college in high pH and thus favour their condensation with reducing sugars. Also, the higher alkyl-pyrazines in the dry aged beef could exist related to the increase of lipid oxidation in it during ageing, as Ha, et al. [3] showed that the dry aged beef had significantly higher TBARS than the wet aged beef at day 56 (p < 0.05). In addition to the Maillard reaction, the alkyl-pyrazines are known to be formed in the lipid-Maillard interaction [ix,29]. The aldehydes formed in lipid oxidation could compete with the dicarbonyl formed from carbohydrate for the amino acids to form alkyl-pyrazines [29]. Alkyl- pyrazines are well known to impart meaty and roasty flavour in cooked beef [viii,nine]. Therefore, the effect of ageing on pH or lipid oxidation could be translated to deviation in volatiles compounds including pyrazines and subsequently modify the flavour attribute of beefiness. The higher pH of dry aged beefiness has been reported in other studies [34,35]. Aside from alkyl-pyrazines, ageing fourth dimension or ageing method significantly impacted on many other Maillard-derived volatiles such as pyridines and pyrroles although these compounds were detected at relatively depression concentrations.
3.2. Gas Chromatography-Olfactometry
The average aromagram for the wet and dry aged grilled beef samples at 35-days ageing are shown in Figure 1 too every bit the trained console description for key peaks, and odour intensity of volatile compounds eluted at dissimilar retention times (Effigy 1). Although for most aroma peaks, only small differences were measured between ageing methods, the odour intensity of several compounds were college in the dry out aged samples. Specifically, the aromagram of dry aged beefiness is characterised by higher bawdy (acetone, 2-methylpropnanal), meaty (iii,v-diethyl-2-methyl-pyrazine), barbecue ((E)-2-nonenal), roasted (2-acetyl-ii-thiazoline), and fatty (p-cresol) odours. The master grilled beefiness olfactory property peaks correspond with the Maillard-derived alkyl-pyrazines, which are well-known components of beef smell [36]. The aromagram was similar to that previously reported in beef [8]. The higher intensity of these odour-active volatiles in dry anile beef supports the higher flavour liking score in the sensory assessment using associated samples [iii]. No off-flavours were detected in the odour profiles of both ageing methods.
3.iii. Relationship betwixt Flavour Liking and Volatiles of Aged Beef
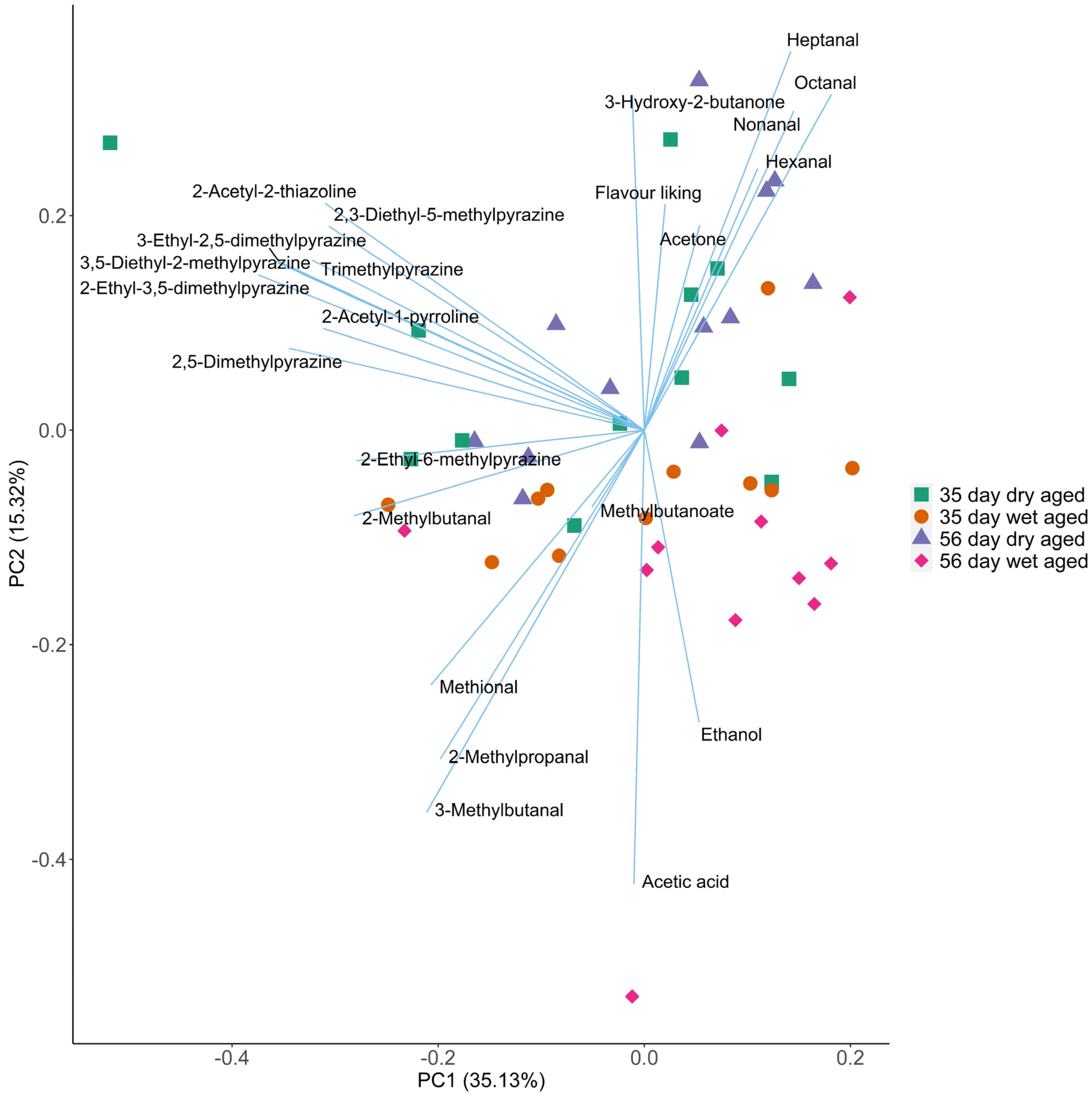
Multivariate assay was conducted on the GC-MS volatiles and consumer flavour liking data previously reported in Ha, et al. [3] to investigate the association between volatiles and consumer season liking of aged beef. Volatile profiles of aged beef and their flavour liking scores are summarised by the PCA biplot (Effigy 2). The result of the KMO test indicated that the selected variables are appropriate for PCA (KMO = 0.67). Although at that place was variance betwixt carcasses within each treatment group, the effects of ageing method and ageing fourth dimension on volatile contour of beef are clearly presented in the biplot. Beef aged for dissimilar periods are separated forth PC1 and PC2. With increased ageing time, the concentrations of most odor touch on volatiles decreased. The products of Maillard reaction and Strecker degradation (eg.2-acetyl-1-pyroline and alkyl-pyrazines) decreased significantly with ageing time afterward 35 days (p < 0.05), whereas a small-scale group of lipid oxidation products (heptanal, decanal) and ethanol increased (p < 0.05). The report of Watanabe, et al. [13] showed that most volatiles derived from Maillard, Strecker degradation, and lipid oxidation increased significantly during the wet ageing of beef M. biceps femoris for 2–30 days, indicating that the effect of ageing time on cooked beef volatiles could differ between cuts. A study on dry out aged Hanwoo longissimus thoracis et lumborum showed a pregnant increase of volatile concentrations from day 0 to twenty-four hour period forty, however, the modify of volatile concentration subsequently day 40 was non reported [11]. In the present study, the decline in volatile concentration with increased ageing fourth dimension (35 vs. 56 days) is reflected by the subtract of flavour liking scores over the same time menses in both dry out and wet aged beef samples [3]. PC1 andPC2 also distinguished the volatile profiles of dry aged beef from its wet anile counterpart. The dry anile beef generally contained higher concentrations of odour impact volatiles compared to that of wet anile beefiness. This finding agrees with previous studies in beef ageing. Male monarch, et al. [10] reported that 14-day dry out anile beef exhibited higher concentrations of nearly volatiles than that in wet aged beef, except for total aldehydes and ketones. Among the 4 combinations of ageing methods and time (wet aged 35 days and 56 days; and dry aged 35 days and 56 days), the 35-day dry aged beefiness was characterised past the highest concentration of many odour bear upon volatiles including 2-ethyl-6-methylpyrazine, 2-ethyl-v-methyl pyrazine, iii-hydroxy-ii-butanone, 2-acetyl-2-thiazoline and trimethyl pyrazine, explaining the high flavour liking scores in the consumer sensory cess [3]. According to Kilgannon, et al. [37], the concentration of many of these volatiles are positively correlated to the flavour liking of wet aged beefiness measured using an MSA consumer sensory console. Therefore, 35-day dry ageing led to a volatile profile with an increment in desirable volatiles preferred by consumers.
To better understand the result of volatiles on season liking of dry/wet aged beef, a PLS regression model was established using flavour liking of aged beef samples as Y and their corresponding concentration of selected volatiles every bit X (Tabular array 2). A moderate PLS model was obtained using simply one latent factor. Similar to the outcome of PCA, flavour liking was positively correlated to well-nigh volatiles. A positive human relationship was measured between season liking and the concentration of alkyl-pyrazines, which agrees with the finding of Frank, et al. [fifteen]. Frank, et al. [fifteen] reported that two-ethyl-3,5-dimethylpyrazine was an important contributor to the grilled season and flavour impact attributes in lamb, while the trimethylpyrazine was positively related to the aged meat flavour. Alkyl-pyrazines are ordinarily reported to impart desirable flavour attributes such as chocolate and roasted in cooked meat [8,15,38]. Similarly, iii-hydroxy-2-butanone, 2-acetyl-1-pyrroline, and 2-acetyl-2-thiazoline take been reported to positively influence the flavour profile of cooked meat [15,26]. Hence, information technology is postulated that the higher concentration of alkyl-pyrazines, 2-acetyl-ii-thiazoline, and 3-hydroxy-2-butanone, in dry anile beef contributed to its loftier flavour liking in sensory assessment. Additionally, the decline in alkyl-pyrazines and two-acetyl-i-pyrroline with the ageing time could be responsible for the subtract of flavour liking with time of ageing. The flavour liking was negatively correlated to the concentrations of acerb acrid and ethanol, which accumulated in the wet ageing procedure most probable due to microbial fermentation in the nowadays study. Flavour liking score was besides positively correlated to several aldehydes produced in lipid oxidation including hexanal, 2-methylbutanal, and nonanal. This result is similar to those reported past Frank, et al. [xv] and Vocal, et al. [38]. The study of Song, et al. [39] showed that a moderate lipid oxidation is essential for the germination of beefiness flavour in cooking. Nonetheless, in the 56-day dry aged beefiness, the concentration of heptanal and octanal reached a relatively loftier level, which may accept caused a perception of oxidised flavour by consumers and thus reduced the sensory score every bit reported previously studies [seven]. The concentration of 3-hydroxy-two-butanone was positively related to the flavour liking in our study. A similar finding was reported by Legako, et al. [26]. Still, the written report of Stetzer, et al. [18] showed that the three-hydroxy-two-butanone was correlated to the 'livery' off-flavour in aged beef. In summary, the PLSR model farther supports that the volatile profile was an important commuter of the flavour liking of aged beef. However, the model should be interpreted cautiously as it was based on a relatively small ready of observations (n = 4, mean-centred values for treatment combinations) and does not take into consideration the important role of non-volatile flavour compounds such every bit free amino acids, peptides and nucleotides. In improver, the roles of particular volatiles in flavor liking needs to be further confirmed in omission and addition tests equally suggested past Frank, et al. [15].
four. Conclusions
Results from this study indicated that the dry anile and moisture aged beefiness exhibited similar volatile profiles measured by GC-MS and GC-O. The concentrations of several aroma- and season-active volatiles varied with ageing method and ageing time. The difference in flavour liking of aged beefiness could exist explained past their respective volatile profiles. The preferred flavour liking of the dry aged beef samples described in a previous report could be attributed to some differences in volatile profiles, especially alkyl-pyrazines, 2-acetyl-two-thiazoline, and 3-hydroxy-2-butanone. Nevertheless, the increased lipid oxidation and loss of desirable volatiles, due east.k., ii-acetyl-ane-pyrroline and alkyl-pyrazines, in the 56-day ageing tin can lead to the decrease in flavour liking. In moisture aged beef, the aggregating of bacterial fermentation products appeared to exist detrimental to its flavour. Therefore, based on the weather used in this written report, 35-day dry ageing is an optimal aging flow to produce beefiness with a desirable volatile profile and flavour. Prolonged (56-day) ageing should be avoided in both dry ageing and wet ageing to preclude the deterioration of flavour, however further studies should be performed to ostend this.
Author Contributions
Conceptualization, R.D.W. and P.M.; methodology, D.F.; software, Z.L.; validation, Chiliad.H. and D.F.; formal data analysis, Z.50.; investigation, 1000.H.; resource, R.D.W. and P.Chiliad.; data curation, D.F.; writing—original draft preparation, Z.Fifty.; writing—review and editing, Z.L., R.D.W., M.H., and D.F.; visualization, Z.L.; supervision, R.D.West., M.H. and D.F.; projection administration, R.D.W. and M.H.; funding acquisition, R.D.West. and P.Chiliad. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded past Meat and Livestock Australia (Project V.RMH.0035).
Institutional Review Board Argument
The report was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Ethics Committee of Kinesthesia of Veterinary and Agricultural Sciences, The Academy of Melbourne (HAEC 1545786.ane).
Informed Consent Argument
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are bachelor on asking from the corresponding author. The data are not publicly available at this moment because they are part of an ongoing Ph.D. Thesis.
Acknowledgments
Funding from Meat and Livestock Australia is gratefully best-selling. Meat and Livestock Commonwealth of australia had no influence on the experimental design, sample drove and analysis or data estimation of this written report. We would like to thank staff at Greenham Tasmania for their help with sample collection, and Top Cut Foods Aureate Coast for the employ of the meat ageing and boning facilities. Acknowledgement is also fabricated to Steve Bonney, Rod Polkinghorne, Liselotte Pannier, Long Huynh and Rozita Vaskoska for their help with sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nair, Grand.North.; Canto, A.C.; Rentfrow, G.; Suman, South.P. Muscle-specific effect of aging on beef tenderness. LWT 2018, 100, 250–252. [Google Scholar] [CrossRef]
- Frank, D.; Zhang, Y.; Li, Y.; Luo, X.; Chen, X.; Kaur, M.; Mellor, G.; Stark, J.; Hughes, J. Shelf life extension of vacuum packaged chilled beef in the Chinese supply chain. A feasibility study. Meat Sci. 2019, 153, 135–143. [Google Scholar] [CrossRef]
- Ha, Yard.; McGilchrist, P.; Polkinghorne, R.; Huynh, L.; Galletly, J.; Kobayashi, K.; Nishimura, T.; Bonney, S.; Kelman, K.R.; Warner, R. Furnishings of different ageing methods on colour, yield, oxidation and sensory qualities of Australian beefiness loins consumed in Australia and Nippon. Food Res. Int. 2019, 125, 108528. [Google Scholar] [CrossRef]
- Lepper-Blilie, A.N.; Berg, E.P.; Buchanan, D.S.; Berg, P.T. Effects of post-mortem aging time and type of aging on palatability of depression marbled beef loins. Meat Sci. 2016, 112, 63–68. [Google Scholar] [CrossRef]
- Terjung, Northward.; Witte, F.; Heinz, V. The dry out aged beef paradox: Why dry aging is sometimes not improve than wet aging. Meat Sci. 2021, 172, 108355. [Google Scholar] [CrossRef]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, M.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking backdrop, and sensory attributes during dry aging of beefiness from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- Campo, M.Grand.; Nute, G.R.; Hughes, S.I.; Enser, M.; Forest, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Brawl, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Mottram, D.Southward. Flavor formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- King, M.-F.; Matthews, M.A.; Rule, D.C.; Field, R.A. Effect of Beef Packaging Method on Volatile Compounds Adult by Oven Roasting or Microwave Cooking. J. Agric. Food Chem. 1995, 43, 773–778. [Google Scholar] [CrossRef]
- Utama, D.T.; Kim, Y.J.; Jeong, H.S.; Kim, J.; Barido, F.H.; Lee, South.K. Comparing of meat quality, fat acid limerick and aroma volatiles of dry out-aged beefiness from Hanwoo cows slaughtered at threescore or 80 months old. Asian-Australas. J. Anim. Sci. 2020, 33, 157–165. [Google Scholar] [CrossRef]
- Frank, D.; Hughes, J.; Piyasiri, U.; Zhang, Y.; Kaur, M.; Li, Y.; Mellor, G.; Stark, J. Volatile and non-volatile metabolite changes in 140-day stored vacuum packaged chilled beefiness and potential shelf life markers. Meat Sci. 2020, 161, 108016. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kamada, Chiliad.; Imanari, M.; Shiba, Due north.; Yonai, M.; Muramoto, T. Issue of crumbling on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.; Shorthose, W.R.; Ferguson, D.M.; Thompson, J.M. Methods used in the CRC programme for the conclusion of carcass yield and beef quality. Aust. J. Exp. Agric. 2001, 41, 953–957. [Google Scholar] [CrossRef]
- Frank, D.; Raeside, K.; Behrendt, R.; Krishnamurthy, R.; Piyasiri, U.; Rose, Yard.; Watkins, P.; Warner, R. An integrated sensory, consumer and olfactometry study evaluating the effects of rearing system and diet on flavor characteristics of Australian lamb. Anim. Prod. Sci. 2017, 57, 347–362. [Google Scholar] [CrossRef]
- Pinheiro, A.C.Grand.; Nunes, C.A.; Vietoris, V. SensoMaker: A tool for sensorial characterization of food products. Ciência E Agrotecnologia 2013, 37, 199–201. [Google Scholar] [CrossRef]
- Resconi, 5.C.; Escudero, A.; Beltrán, J.A.; Olleta, J.L.; Sañudo, C.; Campo, M.D.M. Color, Lipid Oxidation, Sensory Quality, and Aroma Compounds of Beef Steaks Displayed under Different Levels of Oxygen in a Modified Temper Package. J. Nutrient Sci. 2011, 77, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Stetzer, A.J.; Cadwallader, K.; Singh, T.K.; Mckeith, F.Thousand.; Brewer, Grand.S. Effect of enhancement and ageing on flavor and volatile compounds in various beef muscles. Meat Sci. 2008, 79, 13–xix. [Google Scholar] [CrossRef]
- Borch, Due east.; Agerhem, H. Chemical, microbial and sensory changes during the anaerobic cold storage of beef inoculated with a homofermentative Lactobacillus sp. or a Leuconostoc sp. Int. J. Food Microbiol. 1992, 15, 99–108. [Google Scholar] [CrossRef]
- Jones, R.J. Observations on the succession dynamics of lactic acrid bacteria populations in chill-stored vacuum-packaged beefiness. Int. J. Food Microbiol. 2004, 90, 273–282. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D'Souza, Fifty.A.; Teranishi, R.; Buttery, R.Thou. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.Yard. Spoilage of vacuum-packed beef past a clostridium sp. J. Sci. Food Agric. 1989, 49, 473–486. [Google Scholar] [CrossRef]
- El-Magoli, S.B.; Laroia, Due south.; Hansen, P. Flavor and texture characteristics of low fatty footing beef patties formulated with whey protein concentrate. Meat Sci. 1996, 42, 179–193. [Google Scholar] [CrossRef]
- Machiels, D.; Istasse, L.; van Ruth, S.M. Gas chromatography-olfactometry analysis of beef meat originating from differently fed belgian blue, limousin and aberdeen angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Senter, S.D.; Arnold, J.Due west.; Chew, Five. Apc values and volatile compounds formed in commercially processed, raw chicken parts during storage at 4 and 13 °C and nether simulated temperature abuse weather condition. J. Sci. Food Agric. 2000, lxxx, 1559–1564. [Google Scholar] [CrossRef]
- Legako, J.; Dinh, T.; Miller, Chiliad.; Adhikari, K.; Brooks, J. Consumer palatability scores, sensory descriptive attributes, and volatile compounds of grilled beef steaks from three USDA Quality Grades. Meat Sci. 2016, 112, 77–85. [Google Scholar] [CrossRef]
- Mullen, A.G.; Stoeva, South.; Laib, K.; Gruebler, Thou.; Voelter, West.; Troy, D. Preliminary analysis of amino acids at various locations along the Grand. longissimus dorsi in anile beef. Nutrient Chem. 2000, 69, 461–465. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Strecker-type Deposition Produced by the Lipid Oxidation Products iv,5-Epoxy-2-Alkenals. J. Agric. Nutrient Chem. 2004, 52, 7126–7131. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Frank, D.; Watkins, P.; Ball, A.; Krishnamurthy, R.; Piyasiri, U.; Sewell, J.; Ortuño, J.; Stark, J.; Warner, R. Bear upon of Brassica and Lucerne Finishing Feeds and Intramuscular Fatty on Lamb Eating Quality and Flavor. A Cross-Cultural Written report Using Chinese and Non-Chinese Australian Consumers. J. Agric. Food Chem. 2016, 64, 6856–6868. [Google Scholar] [CrossRef]
- Ross, C.F.; Smith, D.M. Use of Volatiles as Indicators of Lipid Oxidation in Musculus Foods. Compr. Rev. Nutrient Sci. Food Saf. 2006, 5, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Bueno, Chiliad.; Escudero, A.; Magalhaes, D.; Ferreira, V.; Campo, M.M. Ageing and retail display time in raw beefiness odour according to the degree of lipid oxidation. Food Chem. 2018, 242, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Madruga, M.S.; Mottram, D.S. The effect of pH on the formation of maillard-derived olfactory property volatiles using a cooked meat system. J. Sci. Nutrient Agric. 1995, 68, 305–310. [Google Scholar] [CrossRef]
- Ahnström, M.L.; Seyfert, M.; Hunt, Thou.C.; Johnson, D.E. Dry crumbling of beef in a bag highly permeable to h2o vapour. Meat Sci. 2006, 73, 674–679. [Google Scholar] [CrossRef]
- Li, X.; Babol, J.; Bredie, Due west.L.P.; Nielsen, B.; Tománková, J.; Lundström, K. A comparative report of beef quality after ageing longissimus muscle using a dry out ageing bag, traditional dry ageing or vacuum package ageing. Meat Sci. 2014, 97, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C.; Grosch, W. Quantification of character-impact aroma compounds of roasted beef. Eur. Nutrient Res. Technol. 1993, 196, 417–422. [Google Scholar] [CrossRef]
- Kilgannon, A.Thou.; Holman, B.Westward.; Frank, D.C.; Mawson, A.J.; Collins, D.; Hopkins, D. Temperature-time combination effects on aged beef volatile profiles and their relationship to sensory attributes. Meat Sci. 2020, 168, 108193. [Google Scholar] [CrossRef]
- Song, S.; Zhang, X.; Hayat, Chiliad.; Huang, M.; Liu, P.; Karangwa, East.; Gu, F.; Jia, C.; Xia, S.; Xiao, Z.; et al. Contribution of beef base to smell characteristics of beeflike process flavour assessed by descriptive sensory assay and gas chromatography olfactometry and partial least squares regression. J. Chromatogr. A 2010, 1217, 7788–7799. [Google Scholar] [CrossRef] [PubMed]
- Song, Due south.; Zhang, 10.; Hayat, 1000.; Liu, P.; Jia, C.; Xia, Due south.; Xiao, Z.; Tian, H.; Niu, Y. Formation of the beefiness flavor precursors and their correlation with chemical parameters during the controlled thermal oxidation of tallow. Food Chem. 2011, 124, 203–209. [Google Scholar] [CrossRef]
Effigy 1. Boilerplate (n = 6 assessors) gas chromatography olfactometry profile of freshly grilled dry out (black) and moisture anile (grey) beef after 35 days.
Figure i. Average (north = half-dozen assessors) gas chromatography olfactometry profile of freshly grilled dry (black) and moisture aged (grey) beef afterwards 35 days.
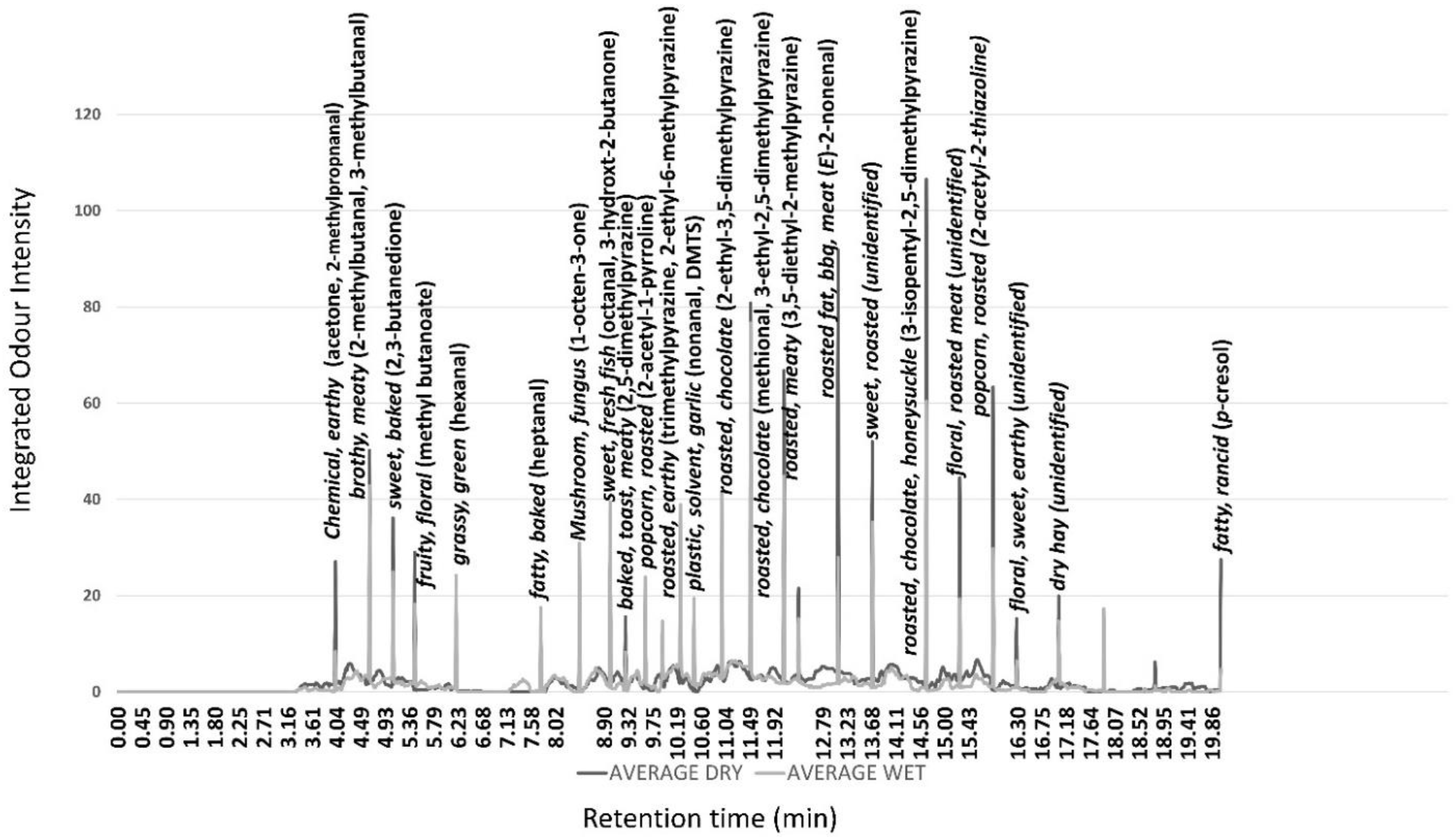
Figure 2. Master component analysis (PCA) biplot showing volatile changes in beef dry aged for 35 days or 56 days, or wet 35 days or 56 days. Values on the axes refer to the variance explained by the first two principal components.
Figure 2. Principal component analysis (PCA) biplot showing volatile changes in beef dry out aged for 35 days or 56 days, or wet 35 days or 56 days. Values on the axes refer to the variance explained by the start ii principal components.
Tabular array 1. Effects of ageing method (AM; Dry aged, Wet aged) and ageing time (AT; 35 days, 56 days) on semi-quantitative data for odour-agile volatiles concentrations (µg/kg) identified in the grilled aged beef headspace analysed with headspace solid phase microextraction-gas chromatography mass spectrometry. Values are ways after aligning for intramuscular fatty (covariate). 4-Methyl-i-pentanol was used as an internal standard for semi-quantification purposes.
Tabular array i. Furnishings of ageing method (AM; Dry anile, Wet anile) and ageing time (AT; 35 days, 56 days) on semi-quantitative data for odour-agile volatiles concentrations (µg/kg) identified in the grilled aged beef headspace analysed with headspace solid phase microextraction-gas chromatography mass spectrometry. Values are ways after adjustment for intramuscular fat (covariate). iv-Methyl-ane-pentanol was used as an internal standard for semi-quantification purposes.
| Volatile Compound | LRI | ID | Odour Descriptors | Lit | m/z | Dry Aged | Wet Anile | SED | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | 56 | 35 | 56 | AM/AT | AM × AT | AM | AT | AM × AT | ||||||
| Alcohols/diols | ||||||||||||||
| Ethanol | EI,R | 12 | 45 | 37 | 55 | 274 | 723 | 84.0 | 118.7 | <0.001 | 0.007 | 0.013 | ||
| i-Pentanol | 945 | EI,R | 12 | 55 | 37.8 | 58.0 | 44.six | 43.3 | 6.23 | viii.82 | 0.529 | 0.136 | 0.091 | |
| one-hexanol | 1354 | EI,R | 12 | 56 | 69 | 105 | 59 | 19 | 31.3 | 44.3 | 0.133 | 0.956 | 0.228 | |
| 1-Heptanol | 1450 | EI,R | 12 | 70 | 21.2 | 43.4 | 20.five | 12.5 | 10.98 | fifteen.53 | 0.156 | 0.525 | 0.177 | |
| 2-Ethylhexanol | 1496 | EI,R | 12 | 57 | 20.iv | 17.7 | 27.8 | 24.ii | iii.11 | 4.39 | 0.030 | 0.315 | 0.885 | |
| ane-Octanol | 1546 | EI,R | 12 | 56 | nine.3 | 17.ii | x.4 | 8.0 | ii.87 | 4.06 | 0.162 | 0.352 | 0.080 | |
| iv-Butoxybutanol | 1701 | EI,R | 57 | 3.93 | 3.44 | iii.79 | 4.66 | 0.69 | 0.98 | 0.444 | 0.785 | 0.334 | ||
| Benzylalcohol | 1900 | EI,R | 108 | 13.iii | 32.3 | 4.three | vi.3 | 4.06 | 5.74 | <0.001 | 0.012 | 0.041 | ||
| 1-Ethylphenol | 2044 | EI,R | 107 | 0.85 | 0.66 | 0.92 | 2.56 | 0.94 | 1.33 | 0.299 | 0.448 | 0.337 | ||
| 2-Ethylphenol | 2194 | EI,R | 107 | 0.51 | 0.34 | 0.45 | 1.59 | 0.66 | 0.93 | 0.364 | 0.465 | 0.322 | ||
| Ketones/diones | ||||||||||||||
| Acetone | 816 | EI,R,O | Chemical, earthy | 43 | 29.6 | 59.7 | 28.v | 48.8 | 8.09 | 11.45 | 0.144 | 0.015 | 0.230 | |
| 2-Butanone | 886 | EI,R | 12 | 72 | 83.1 | 76.four | 76.3 | 59.7 | 6.95 | 9.83 | 0.095 | 0.100 | 0.478 | |
| 2-Pentanone | 975 | EI,R | 86 | 44.1 | 49.2 | 63.four | 69.5 | vii.54 | 10.67 | 0.011 | 0.462 | 0.948 | ||
| two-Heptanone | 1151 | EI,R | eight,xv,xxx | 58 | half dozen.76 | four.98 | iii.31 | 9.67 | i.38 | 1.96 | 0.654 | 0.104 | 0.005 | |
| ii-Octanone | 1238 | EI,R | 12 | 58 | 3.77 | v.63 | ii.54 | 6.31 | one.31 | 1.85 | 0.834 | 0.036 | 0.470 | |
| iii-Hydroxy-2-butanone | 1304 | EI,R,O | Sweet, fresh, fishy | 12,26 | 45 | 203 | 183 | 126 | 143 | 49.eight | 70.5 | 0.033 | 0.305 | 0.530 |
| ii-Methyl-3-octanone | 1322 | EI,R | 99 | three.77 | 5.63 | 2.54 | 6.31 | 1.31 | ane.85 | 0.834 | 0.036 | 0.470 | ||
| 2-Nonanone | 1388 | EI,R | eight,15,thirty | 58 | 2.71 | 6.eighteen | 3.31 | 3.83 | 0.79 | i.12 | 0.275 | 0.015 | 0.067 | |
| i,3-Butanediol | 1600 | EI,R | 45 | 1.5 | 1.six | three.4 | vi.9 | two.02 | 2.86 | <0.001 | 0.095 | 0.101 | ||
| Butyrolactone | 1637 | EI,R | 86 | 24.i | 23.0 | 27.3 | 32.7 | eight.10 | 11.45 | 0.432 | 0.794 | 0.688 | ||
| Pyrazines | ||||||||||||||
| 2-Methylpyrazine | 1285 | EI,R | 8,12,fifteen,30 | 94 | 27.6 | 25.four | xix.6 | 17.iv | iv.47 | 6.32 | 0.081 | 0.624 | 0.999 | |
| 2,5-Dimethylpyrazine | 1330 | EI,R,O | Broiled, toast, meaty | 108 | 100.2 | 82.7 | 78.4 | 55.5 | 14.78 | 21.91 | 0.103 | 0.177 | 0.857 | |
| ii,vi-Dimethylpyrazine | 1338 | EI,R | 8,12,15,30 | 108 | 51.4 | 28.7 | 38.6 | 16.v | seven.85 | 11.10 | 0.117 | 0.006 | 0.972 | |
| 2,three-Dimethylpyrazine | 1346 | EI,R | 8,12,15,30 | 108 | 11.28 | 8.07 | 5.66 | 5.07 | 1.89 | 2.67 | 0.026 | 0.319 | 0.490 | |
| 2-Ethyl-five-methylpyrazine | 1384 | EI,R | Roasted, chocolate | 12 | 121 | 10.73 | 7.23 | 6.66 | 4.32 | 1.02 | 1.44 | 0.001 | 0.006 | 0.571 |
| 2-Ethyl-6-methylpyrazine | 1390 | EI,R,O | Roasted, earthy | 12 | 121 | 19.63 | 14.seventy | 12.54 | 8.13 | 1.73 | two.44 | <0.001 | 0.009 | 0.880 |
| Trimethyl pyrazine | 1410 | EI,R,O | Roasted, earthy | 8,12,15,thirty | 122 | 66.2 | 57.half dozen | 35.7 | 43.0 | 11.eleven | 15.71 | 0.048 | 0.955 | 0.477 |
| 3-Ethyl-2,5- dimethylpyrazine | 1442 | EI,R,O | Roasted, chocolate | 8,12,fifteen,30 | 135 | 23.9 | 13.2 | 11.iv | 9.9 | 3.06 | 4.33 | 0.013 | 0.051 | 0.143 |
| 2-Ethyl-iii,5-dimethylpyrazine | 1469 | EI,R,O | Roasted, chocolate | viii,12,15,30 | 135 | 61.v | 39.0 | 46.1 | 31.9 | 8.45 | 11.94 | 0.010 | 0.015 | 0.404 |
| 3,five-Diethyl-ii- methylpyrazine | 1490 | EI,R,O | Roasted, meaty | 149 | 5.95 | iii.50 | iii.04 | 2.30 | 0.lxxx | ane.thirteen | 0.013 | 0.051 | 0.291 | |
| 2,3-Diethyl-5- methylpyrazine | 1499 | EI,R | 149 | 3.01 | 0.98 | 0.57 | 0.72 | 0.52 | 0.73 | 0.012 | 0.075 | 0.040 | ||
| iii,5-Dimethyl-2-isobutylpyrazine | 1549 | EI,R | 122 | 2.69 | 2.81 | i.94 | one.56 | 0.54 | 0.76 | 0.068 | 0.808 | 0.642 | ||
| Dimethyl isopentylpyrazine | 1655 | EI,R | viii,12,xv,30 | 122 | 19.iii | 23.1 | 20.7 | 15.iii | three.83 | 5.42 | 0.404 | 0.833 | 0.233 | |
| Aldehydes | ||||||||||||||
| 2-Methylpropanal | 804 | EI,R,O | Chemical, earthy | eight,15,30 | 72 | 34.9 | 41.6 | 41.9 | 26.4 | five.15 | 7.28 | 0.427 | 0.393 | 0.035 |
| ii-Methylbutanal | 915 | EI,R,O | Brothy, meaty | 26 | 57 | 328 | 344 | 332 | 264 | 45.2 | 64.0 | 0.402 | 0.571 | 0.355 |
| three-Methylbutanal | 919 | EI,R,O | Brothy, compact | 8, 15,30 | 58 | 113.8 | 119.3 | 142.4 | 111.9 | xv.69 | 22.19 | 0.502 | 0.427 | 0.257 |
| Hexanal | 1078 | EI,R,O | Grassy, light-green | 8,15,26,30 | 56 | 111 | 231 | 138 | 163 | 49.4 | 69.8 | 0.677 | 0.149 | 0.335 |
| Heptanal | 1194 | EI,R,O | Fatty, baked | 8,fifteen,26,30 | seventy | 20.three | 78.two | 34.7 | 41.two | 12.66 | 17.xc | 0.375 | 0.014 | 0.047 |
| Octanal | 1328 | EI,R,O | Sweet, fresh fish | 8,15,26,30 | 84 | 8.0 | 18.ix | xiv.2 | 9.4 | 2.50 | 3.53 | 0.517 | 0.222 | 0.003 |
| Nonanal | 1380 | EI,R,O | Plastic, solvent, garlic | 8,xv,26,30 | 57 | 58.v | 94.1 | 92.1 | 59.5 | 9.98 | xiv.12 | 0.958 | 0.879 | 0.001 |
| Furfural | 1439 | EI,R | eight | 96 | 1.9 | two.1 | 1.eight | 6.1 | ii.15 | 3.04 | 0.376 | 0.311 | 0.343 | |
| Decanal | 1500 | EI,R | 8,12,15,xxx | 57 | 0.19 | 0.70 | 0.31 | 0.61 | 0.xix | 0.27 | 0.943 | 0.036 | 0.567 | |
| Benzaldehyde | 1508 | EI,R | 8,12,15,30 | 105 | 57.1 | 52.9 | 71.two | 81.3 | 8.33 | 11.78 | 0.014 | 0.725 | 0.397 | |
| 2,5-Dimethylbenzaldehyde | 1705 | EI,R | 134 | 177 | 70 | 210 | 169 | 27.2 | 38.v | 0.019 | 0.009 | 0.224 | ||
| four-Ethylbenzaldehyde | 1732 | EI,R | 134 | 2.41 | 2.85 | 1.96 | 5.35 | i.46 | two.07 | 0.486 | 0.196 | 0.319 | ||
| Long chain aldehyde | 1736 | EI,R | 57 | 38 | 21 | 56 | 73 | vii.6 | ten.eight | 0.001 | 0.924 | 0.052 | ||
| Sulphur compounds | ||||||||||||||
| Dimethyl disulphide | 1084 | EI,R | eight,12,15,thirty | 94 | 21.2 | four.1 | 8.7 | 8.4 | 4.54 | half-dozen.42 | 0.374 | 0.059 | 0.069 | |
| Dimethyl trisulphide | 1266 | EI,R,O | Plastic, solvent, garlic | 8,30 | 79 | 23.1 | 0.ane | 0.ix | 0 | 10.48 | 20.97 | 0.291 | 0.260 | 0.296 |
| Methional | 1447 | EI,R,O | Roasted, chocolate | 8,15,30 | 76 | 1.93 | ii.16 | 3.66 | two.55 | 0.63 | 0.89 | 0.099 | 0.484 | 0.289 |
| Methionol | 1717 | EI,R | 106 | 0.83 | 0.53 | 1.17 | 0.82 | 0.28 | 0.40 | 0.263 | 0.260 | 0.934 | ||
| ii-Acetyl-2-thiazoline | 1756 | EI,R,O | Popcorn, roasted | 8,15,30 | 129 | 1.31 | 1.14 | 0.75 | 0.14 | 0.22 | 0.31 | 0.001 | 0.091 | 0.333 |
| Benzothiazole | 1955 | EI,R | 135 | 43 | 32 | 38 | 91 | 32.0 | 45.2 | 0.411 | 0.512 | 0.319 | ||
| Acids | ||||||||||||||
| Acetic acid | 1461 | EI,R | 12 | 60 | 38 | 34 | 104 | 148 | 24 | 34 | <0.001 | 0.412 | 0.317 | |
| Esters | ||||||||||||||
| Methyl butanoate | 978 | EI,R,O | Fruity, floral | viii,15,30 | 74 | 76.8 | 58.5 | 78.5 | 64.6 | 7.43 | ten.50 | 0.603 | 0.035 | 0.769 |
| Butyl formate | 996 | EI,R | 56 | 147.0 | 109.8 | 148.iv | 112.0 | 11.99 | 16.96 | 0.881 | 0.003 | 0.972 | ||
| Methyl-2-methylbutanoate | 1008 | EI,R | 88 | 20.37 | 14.14 | 19.45 | 16.07 | 1.92 | 2.72 | 0.795 | 0.016 | 0.461 | ||
| Ethyl nonanoate | 1520 | EI,R | 74 | iv.53 | 6.62 | 5.67 | v.53 | one.04 | 1.47 | 0.981 | 0.354 | 0.291 | ||
| Methyl salicylate | 1747 | EI,R | 120 | iv.7 | 4.7 | 4.two | 17.1 | six.55 | ix.26 | 0.368 | 0.328 | 0.328 | ||
| Others | ||||||||||||||
| Pyridine | 1204 | EI,R | 79 | 31.7 | 31.3 | 24.six | 18.0 | 2.81 | 3.98 | <0.001 | 0.214 | 0.270 | ||
| 2-Pentylfuran | 1250 | EI,R | 8,15,30 | 81 | 2.93 | 10.32 | iii.06 | 7.98 | ane.54 | two.17 | 0.474 | <0.001 | 0.425 | |
| Pyrrole | 1524 | EI,R | 67 | five.04 | 3.68 | 3.97 | 5.18 | 0.74 | 1.05 | 0.965 | 0.727 | 0.0052 | ||
| 2-Acetyl-one-pyrroline | 1998 | EI,R,O | Popcorn, roated | viii,fifteen,30 | 94 | 8.55 | 5.58 | vii.93 | four.20 | 0.78 | 1.11 | 0.205 | <0.001 | 0.628 |
Table 2. Estimated regression coefficients from partial least squares models for prediction of season liking using concentrations of selected odour-active volatiles in anile beef. Models based on i latent variable or factor.
Table 2. Estimated regression coefficients from partial to the lowest degree squares models for prediction of flavour liking using concentrations of selected odour-active volatiles in aged beef. Models based on one latent variable or gene.
| Variables | Regression Coefficient |
|---|---|
| Constant | 69.9888 |
| ii-Ethyl-6-methylpyrazine | 0.3508 |
| ii,5-Dimethylpyrazine | 0.3466 |
| ii-Ethyl-3,5-dimethylpyrazine | 0.344 |
| ii-Acetyl-1-pyrroline | 0.3343 |
| iii,v-Diethyl-ii-methylpyrazine | 0.3189 |
| 3-Ethyl-2,5-Dimethylpyrazine | 0.317 |
| 2-Acetyl-2-thiazoline | 0.2976 |
| 2-Methylbutanal | 0.2880 |
| two,3-Diethyl-5-methylpyrazine | 0.2820 |
| Butylformate | 0.2718 |
| Ethanol | −0.2631 |
| Acetic acid | −0.2569 |
| 3-Hydroxy-2-butanone | 0.2556 |
| ane-Hexanol | 0.2547 |
| Methylbutanoate | 0.2314 |
| 2-Methylpropanal | 0.1713 |
| Acetone | −0.1299 |
| Dimethyl trisulfide | 0.1238 |
| Heptanal | −0.0908 |
| Nonanal | 0.0764 |
| Octanal | −0.045 |
| Hexanal | 0.0442 |
| s.d. | 4.61 |
| Osten'due south F-test | <0.001 |
| RMSECV | 2.31 |
| Correlation coefficient | 0.94 |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This commodity is an open up access article distributed under the terms and weather condition of the Artistic Eatables Attribution (CC BY) license (https://creativecommons.org/licenses/past/iv.0/).
threlkeldneen1997.blogspot.com
Source: https://www.mdpi.com/2304-8158/10/12/3113/htm